Citric Acid Ionic or Covalent
Types of Compounds. Covalent compounds have weak force of attraction between their molecules so they are usually liquids or gases.

Formation Of An Ionic Bond Between The Precationized Cotton And Citric Download Scientific Diagram
In particular the biosensor did not show any appreciable loss in activity even when stored at 4 for 20 days.

. PH of Acids and Bases. Heat protection up to 450F232C Against grooming breakage vs untreated. Download Full PDF Package.
The hydrogen bond has a wide transition zone which continuously merges with the covalent bond van der Waals and ionic interaction. Inorganic compounds are those derived primarily from non-living sources such as rocks and minerals. You have arrived exactly at the right place.
Pure acids without water are composed of covalent small molecules. Each atom will carry a charge from the transfer of electrons. A Enzymes are nonspecific b Enzymes speed up the rates of chemical reactions c Enzymes require a lot of energy to synthesize d Enzymes are not important in biological systems E Reactants in enzyme-catalyzed reactions are called substrates F Enzymes lower.
Acids are chemical opposites of bases and bases are hydrogen ions that accept hydrogen ions. Theorys flaws is that it doesnt account for acid-base reactions that dont involve the development of a coordinate covalent bond. Due to the mobile ions present acids diluted with water are considerably better conductors.
Chemical compounds are pure substances that are made up of two or more elements that are chemically combined in fixed mass ratios. Choose all that apply. Examples of strong acids are hydrochloric acid HCℓ sulphuric acid H 2 SO 4 and nitric acid.
An acid-base reaction occurs whenever an acid donates a. The second phase occurs by the reversal of the first phase reactions and is initiated by formation of a Schiff base with the α-keto acid substrate and pyridoxamine phosphate. Group of answer choices.
The response by the biosensor presented a linear relationship in the range of 00110 mM. Is a substance that produces hydrogen ions H hydronium ions H 3 O when it dissolves in water Brønsted-Lowry theory. These are organic compounds and inorganic compounds.
Wiley International Edition _Unit Operations CORRECTED SECOND PRINTING. Is a proton H ion donor. The same type of interaction Lanthanum-modified chitosan oligosaccaharides Cos-La nanocontainers were prepared by simple ionic cross linking between oligosaccaharides and Lanthanum-citric acid complex.
Which of the following statements about enzymes are true. Acid phosphatase was immobilized by covalent linkage and entrapment in glutaraldehyde-crosslinked gelatin. As emphasized at the beginning of this chapter very similar chemiosmotic mechanisms are.
Based on the origin compounds can be classified into two types. Ionises completely in water to form a high concentration of H 3 O ions. On the basis of the DFT calculations table S1 P-T generally has quadruple hydrogen bonds with interaction energy of 6836 kcalmol and the averaged interaction energy 1709 kcalmol is within the range of hydrogen bond of.
As the term describes itself general engineering is the branch of science and technology that deals with many areas of science such as electrical mechanical chemical architectural civil and. Are you a student of engineering and looking for the largest collection of engineering quiz questions for a practice test. Acid Arrhenius theory.
The NH 2 group from the amino acid is transferred to pyridoxal phosphate with formation of the corresponding α-keto acid. Download Free PDF Download PDF Download Free PDF View PDF. Hydrogen ionic and covalent.
When water is added the pure acid molecules dissociate in water to form ions commonly referred to as the ionized acid Pure acids are poor conductors of electricity. Glycolysis Pyruvate dehydrogenase complex Citric acid cycle electron transport chain fermentation. Reduces visible signs of hair damage including split ends while preventing breakage future damage.
A few examples of inorganic compounds are common salt marble washing soda baking soda. New Terephthalic Acid Process. Light-dependent reactions Calvin cycle.
Download Free PDF Download PDF Download Free PDF View PDF. What represents a formula for a chemical compound. I a Ionic compounds have strong force of attraction between the oppositely charged ions eg Na and Cl so they are solids.
Which of the following statements is true of the bonds in a water molecule. Builds all three types of bonds in the hair. The capacity to explain the acidic or basic character of ionic species is one advantage of the Bronsted-Lowry definition of acids and bases.
In doing so we also accomplish a larger purpose. Compounds such as the citric acid in lemon juice the ethanoic acid in vinegar or a typical laboratory acid like hydrochloric acid all give their hydrogen ions away in chemical reactions known as acid-base reactions. In chemistry the empirical formula of a chemical is a simple expression of the relative number of each type of atom or ratio of the elements in the compound.
Therefore electrostatic interaction and hydrogen bonds played an important role in the loading of active herbicide in optimal-PCMs-SS carrier. Empirical formulae are the standard for ionic compounds such as CaCl 2 and for macromolecules such as SiO 2An empirical formula makes no reference to isomerism structure or absolute number of atoms. The electron in each hydrogen atom is completely transferred to the oxygen atom and each hydrogen atom has a net charge of 1.
Mechanism of the first phase of transamination. To find the numeric value of the acidity or basicity level of a substance the pH scale. Having considered in general terms how a mitochondrion uses electron transport to create an electrochemical proton gradient we need to examine the mechanisms that underlie this membrane-based energy-conversion process.
B Ionic compounds are soluble in water but covalent compounds are insoluble in water. Mitosis chromosome haploid diploid polyploidy prophase metaphase anaphase cytokinesis meiosis. In addition it has been reported that microbial.

7 Mind Blowing Chemical Reactions You Won T Believe Are Real Chemical Reactions Mind Blown Chemical Changes
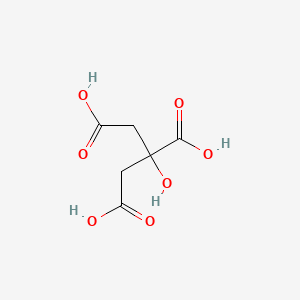

No comments for "Citric Acid Ionic or Covalent"
Post a Comment